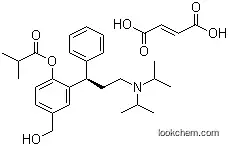
286930-03-8Relevant articles and documents
ANTIMUSCARINIC COMPOUND HAVING A LOW CONTENT OF IMPURITIES
-
Paragraph 0061, (2016/02/20)
Substantially stable to degradation Fesoterodine fumarate, a process for its preparation and a process for the synthesis of specific degradation impurities of Fesoterodine fumarate are disclosed.
PROCESS FOR THE PREPARATION OF OPTICALLY PURE FESOTERODINE DERIVATIVES
-
Paragraph 0240-0242, (2015/04/15)
3,3-diphenylpropylamines of general formula (I), particularly Fesoterodine, as well as their enantiomers, solvates and salts, can be produced by treating a compound of formula (II) with a chiral alcohol to yield the diastereomeric esters of formula (IV) and (IV′), which can be further transformed into a compound of formula (I), or an enantiomer, solvate or salt thereof, wherein R1 is C1-C8 alkyl; and R2 and R3, independently of one another, represent H or C1-C6 alkyl, or together form a ring of 3 to 7 members with the nitrogen to which they are bound.
PROCESS FOR THE PREPARATION OF 2-(3-N,N-DIISOPROPYLAMINO-1-PHENYLPROPYL)-4-HYDROXYMETHYL-PHENOL AND ITS DERIVATIVES
-
, (2014/02/15)
The invention concerns a process for the preparation of 2-(3-N,N- diisopropylamino-l-phenylpropyl)-4-hydroxymethyl-phenol and its derivatives, particularly the corresponding (R) 4-trityloxymethyl derivative, useful as intermediate form in the synthesis of Fesoterodine and its salts, in particular for the preparation of Fesoterodine fumarate salt.
PROCESS FOR THE PREPARATION OF FESOTERODINE OR ITS SALTS
-
, (2013/04/13)
The present invention relates to a process for the preparation of fesoterodine or its salts.
PROCESS FOR THE PREPARATION OF OPTICALLY ACTIVE 3,3-DIPHENYLPROPYLAMINES
-
, (2013/08/15)
The invention relates to a process for obtaining 3,3-diphenylpropylamines of general formula (I), particularly Fesoterodine, as well as their enantiomers, solvates and salts, comprising treating a compound of formula (II) with a chiral alcohol to yield the diastereomeric esters of formula (IV) and (IV'), which can be further transformed into a compound of formula (I), or an enantiomer, solvate or salt thereof, wherein R1 is C1-C8 alkyl; and R2 and R3, independently of one another, represent H or C1-C6 alkyl, or together form a ring of 3 to 7 members with the nitrogen to which they are bound.
A PROCESS FOR PREPARING FESOTERODINE
-
, (2012/10/18)
The present invention relates to an improved process for the preparation of Fesoterodine and pharmaceutically acceptable salts thereof. The present invention particularly relates to a process for the preparation of fesoterodine and pharmaceutically acceptable salts thereof which involves use and preparation of R(+) benzyl tolterodine and fumarate salt of R(+)-[4-benzyloxy-3-(3-diisopropylamino-1-phenylpropyl)-phenyl]-methanol.
PROCESS FOR THE PREPARATION OF MUSCARINIC RECEPTOR ANTAGONIST
-
, (2012/08/07)
The present invention relates to novel and improved processes for the preparation of (r)-2-(3-(diisopropylamino)-1-phenylpropyl)-4-(hydroxymethyl)phenylisobutyrate represented by the following structural formula-1 and its pharmaceutically acceptable salts thereof.
PROCESSES FOR THE PREPARATION OF FESOTERODINE
-
, (2012/03/26)
The invention relates to improved process for the preparation of fesoterodine and its pharmaceutically acceptable salt, specifically fesoterodine fumarate of formula (1). The invention relates to solid state forms of a novel salt of fesoterodine and process for the preparation thereof. The invention also relates to highly pure fesoterodine fumarate substantially free of impurity X at RRT 1.37. The invention also provides solid particles of pure fesoterodine fumarate wherein 90 volume-percent of the particles (D90) have a size of higher than 200 microns.
PROCESS FOR PREPARATION OF PHENOLIC MONOESTERS OF HYDROXYMETHYL PHENOLS
-
, (2011/11/30)
A process for the preparation of phenolic monoesters of 2-(3-diisopropylamino-1-phenylpropyl)-4-(hydroxymethyl)phenol by converting (±)6-halo-4-phenylchroman-2-one to (±)4-halo-2-(3-hydroxy-1-phenylpropyl)phenol. The two hydroxyl groups are protected and the protected compound is reacted with diisopropylamine to give (±)[3-(2-benzyloxy-5-halophenyl)-3-phenylpropyl]diisopropylamine. The halo substituent on the benzene ring is converted to corresponding benzyl alcohol and then the protection is removed to give racemic 5-HMT. Racemic 5-HMT is converted R enantiomer and then it is esterified.
The lactol route to fesoterodine: An amine-promoted Friedel-Crafts alkylation on commercial scale
Dirat, Olivier,Bibb, Andrew J.,Burns, Colin M.,Checksfield, Graham D.,Dillon, Barry R.,Field, Stuart E.,Fussell, Steven J.,Green, Stuart P.,Mason, Clive,Mathew, Jinu,Mathew, Suju,Moses, Ian B.,Nikiforov, Petar I.,Pettman, Alan J.,Susanne, Flavien
, p. 1010 - 1017 (2011/12/16)
We report the discovery and optimization of an amine-promoted Friedel-Crafts alkylation of cinnamaldehyde with 4-hydroxymethyl phenol. This reaction has been used successfully on commercial scale (200 kg) in the context of the manufacture of fesoterodine, a muscarinic antagonist used for the treatment of overactive bladder. Reductive aminations of diisopropylamine and lactol 4 are also discussed, as well as the resolution of the racemic amine rac-2 into its enantiomerically pure form.